Russia coronavirus vaccine has no serious adverse effects — Lancet study

The Lancet, one of the world’s oldest peer-reviewed medical journal based in New York and Beijing, has revealed in a report published on Friday September 4, 2020, that early results from trials of Russia’s potential coronavirus vaccine showed no major negative effects.
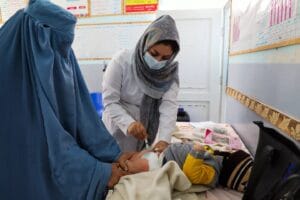
According to the journal’s article, doctors who were involved in the trials used 76 healthy volunteers from age 18 to 60 in the studies that were conducted at two hospitals in Russia.

It was also stated that the vaccine formulations tested were safe and well tolerated.
Two open, non-randomized phase 1/2 studies at two hospitals in Russia,” on 76 healthy volunteers aged 18 to 60.
According to the author of the study, no serious adverse effects were found on the participant and the vaccine creates an antibody response.
“The two 42-day trials – including 38 healthy adults each – did not find any serious adverse effects among participants, and confirmed that the vaccine candidates elicit an antibody response,” the article reads.
The study though suggested that further monitory is needed to ensure safety and effectiveness of the vaccine.
“Large, long-term trials including a placebo comparison, and further monitoring are needed to establish the long-term safety and effectiveness of the vaccine for preventing COVID-19 infection,” it adds.
The chief of Russian sovereign wealth fund RDIF, Kirill Dmitriev, in an interview with CBNC applauded the report by Lancet noting that the report has just validated Russia’s efforts in the production of the vaccine,” Dmitriev says.
“We had lots of interest in the Russian vaccine (with) publication in the Lancet, which is one of key Western magazines on medicine.
“It is very important to share information with the world.
The results have been very good but basically the study showed there is very strong both antibodies and cell immune response,” he added.
He further said that the country plans to release phase 3 human trials by the end of November. He added that there would be clinical trials in other countries such as UAE, and Saudi Arabia.
“Right now we have 40,000 clinical trials going on in Russia, we started it at the end of August, and there will also be clinical trials in the UAE, Saudi Arabia, Philippines and many other markets,” he said.
“So basically we’re on track to have registration not only available in Russia … but also available to key other countries already around November,” added Dmitriev.