Health officials modify monkeypox vaccine authorization to stretch supply fivefold
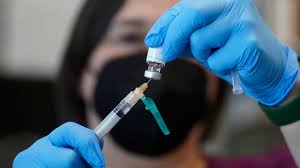
Federal health officials has announced a new strategy to administer the monkeypox vaccine, a move they say will increase the supply fivefold.
The FDA modified its emergency use authorization to allow health providers to administer just 0.1ml of the Jynneos monkeypox vaccine intradermally – between layers of the skin – instead of injecting 0.5ml of the vaccine subcutaneously – under the skin – allowing for five doses of the shot in a single vial.

“This is very welcome news in our fight against monkeypox,” Health and Human Services secretary Xavier Becerra said at a briefing on Tuesday. “Today’s action will boost and strengthen our response further. It safely accelerates and multiplies our supply of effective vaccine by up to fivefold.”
CDC Director Dr. Rochelle Walensky said that under the new plan, the 441,000 vaccine doses in the federal stockpile could stretch to 2.2 million. White House monkeypox coordinator Bob Fenton said at the briefing that the 150,000 doses of the vaccine set to arrive in September could be as many as 750,000 – which, with two vaccine doses per person, could vaccinate roughly 365,000 people.
Fenton called the new strategy a “game-changer,” adding that the plan would help the country “stay ahead of the virus” without compromising safety or effectiveness.
“It’s safe, it’s effective, and it will significantly scale the volume of vaccine doses available for communities across the country,” he added.
FDA Commissioner Dr. Robert Califf touted data from a climnical study of Jynneos prior to its approval in 2019, which evaluated intradermal administration of the vaccine compared to subcutaneously.
“Individuals who received the vaccine intradermally received a lower volume – one-fifth – than individuals who received the vaccine subcutaneously,” he explained. “The results of this study demonstrated that intradermal administration produces similar immune response to subcutaneous administration. Many individuals in both groups responded to vaccination in a similar way.”
Dr. Califf urged those who have the opportunity to get vaccinated with the Jynneos vaccine “to consider getting your first dose immediately.”
This intradermal strategy is for adults only. The emergency use authorization also allows for children who are at high risk of monkeypox infection to receive the vaccine subcutaneously.
The highly unusual step is a stark acknowledgment that the U.S. currently lacks the supplies needed to vaccinate everyone seeking protection from the rapidly spreading virus. That includes 1.6 million to 1.7 million Americans considered by federal officials to be at highest risk from the disease, primarily men with HIV or have a higher risk of contracting it. Vaccinating that group would require about three times more full doses than the roughly 1.1 million officials have made available.
Some experts and advocates warned there is little data to support the policy and worried it could backfire if it reduces the vaccine’s effectiveness.
“We have grave concerns about the limited amount of research that has been done on this dose and administration method, and we fear it will give people a false sense of confidence that they are protected,” said David Harvey of the National Coalition of STD Directors, in a statement.
The Biden administration declared monkeypox a public health emergency last week in an effort to slow the growing outbreak that has infected more than 8,900 Americans.
According to HHS, more than 600,000 doses of Jynneos have been shipped to states and jurisdictions nationwide as of Monday. The department says more than 1 million have been made available to local and state health departments so far, and roughly 1.5 million people are eligible for vaccination.